Further Steps for Manufacturing or Importing a Medical Device for Commercial Purposes in Thailand
(Before proceeding, make sure you've read Steps 1–2 to check product classification and identify the purpose of import/manufacture.)
Step 3 : Establishment Licensing
Anyone who wishes to manufacture or import medical devices for commercial purposes must register their establishment with the Thai FDA.
Fee
Application fee | Inspection fee | Certificate fee | Total | |
Manufacturer Establishment License | 100 THB | 12,000 THB | 2,000 THB | 14,100 THB |
Importer Establishment License | 100 THB | 12,000 THB | 4,000 THB | 16,100 THB |
Please click here for more information (in Thai)
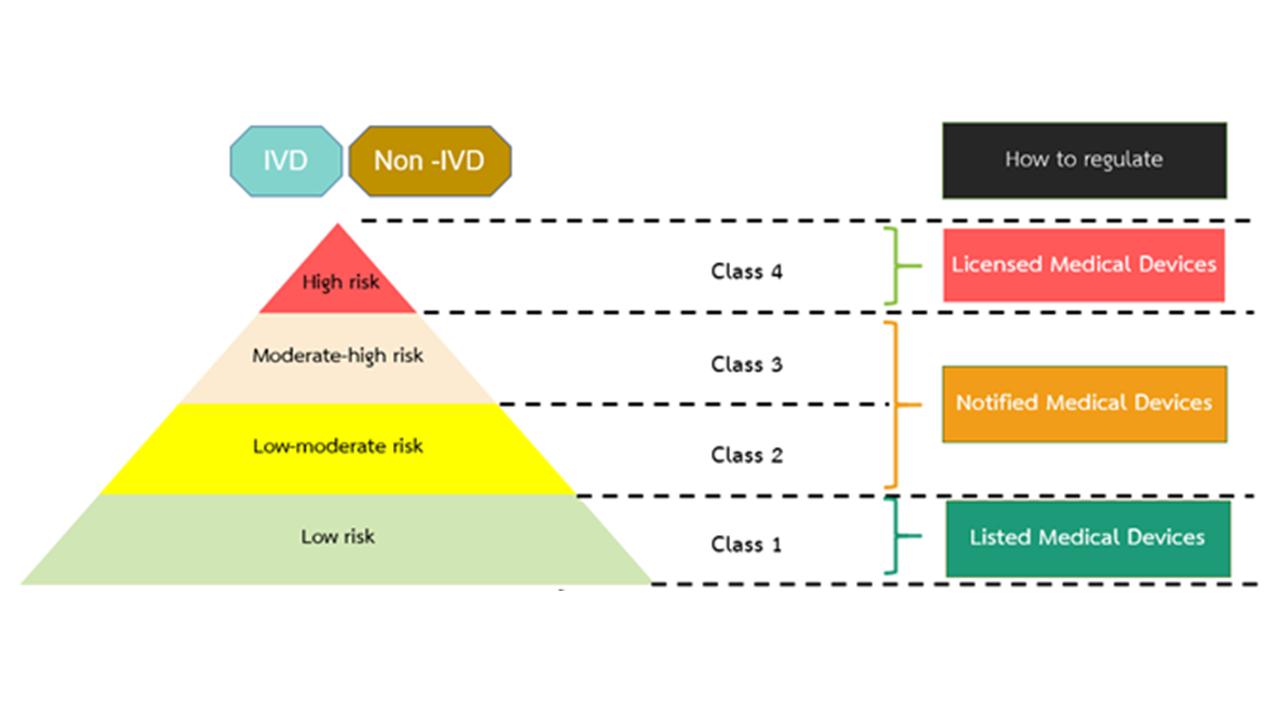
Step 4 : Determination of Medical Device Risk Classification
To ensure effective regulation, medical devices in Thailand are classified into four risk-based categories, as shown in the diagram below.
This classification aligns with ASEAN Medical Device Directive (AMDD) to ensure safety and performance, and to protect consumers and patients.
An establishment registrant who wishes to register a medical device in Thailand must first determine its risk classification, as registration requirements vary by risk class.
You can check your devices' risk classification here by answering a few questions
Step 5 : Medical Device Registration
Registration requirements for each classification are shown in the table below.
Submission Dossier for Class 1 Medical Devices |
Medical Device Label |
Instruction for Use (IFU) |
Product Specification |
Device Description and Features |
Materials |
Declaration of conformity |
Regulatory Approval History (If any) |
Sterilization Report (For Sterilized Medical Devices) |
Calibration Report (For Medical Devices with Measuring Function) |
List of Medical Devices and Grouping (In case of Grouped Medical Devices Application) |
Letter of Authorization |
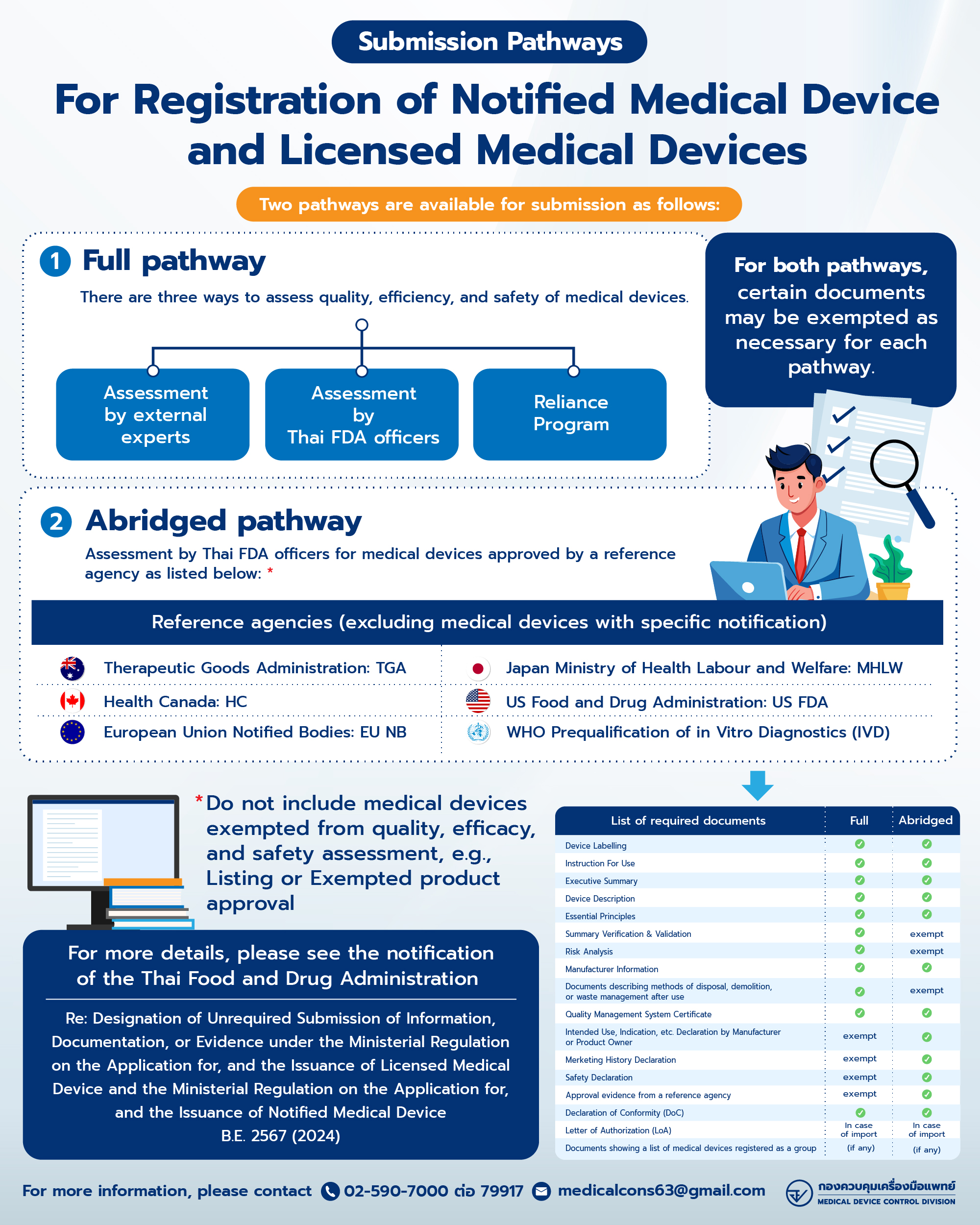
Submission Dossier for Class 2-4 Medical Devices | |||
Full | Abridged | Reliance with HSA | |
Medical Device Label | / | / | / |
Instruction for Use (IFU) | / | / | / |
Executive Summary | / | / | / |
Device Description | / | / | / |
Essential Principles | / | / | / |
Summary verification & validation | / | - | / |
Risk Analysis | / | - | / |
Manufacturer Information | / | / | / |
Document Describing Methods of Disposal, Demolition, or Waste Management after Use | (if any) | - | (if any) |
Quality Management System Certificate | / | / | / |
Declaration of conformity | / | / | / |
List of Medical Devices and Grouping (In case of Grouped Medical Devices Application) | (if any) | (if any) | (if any) |
Intended Use, Indication etc. Declaration | - | / | - |
Market History Declaration | - | / | - |
Safety Declaration | - | / | - |
Approval Evidence from Reference Agency | - | / | - |
Letter of Authorization | / | / | / |
Change Notification Approved from HSA | - | - | / |
Regulatory Reliance Participating Letter | - | - | / |
Approval Evidence from HSA | - | - | / |
Consent form | - | - | / |
Fee
Application fee | Document | Expert fee | Certificate fee | Total | |
Certificate of Listed Medical Device (Class 1) | |||||
Local Manufacture | 500 THB | 300 THB | - | 1,000 THB | 1,800 THB |
Import | 500 THB | 600 THB | - | 2,000 THB | 3,100 THB |
Certificate of Notified Medical Device (Class 2-3) | |||||
Local Manufacture | 1,000 THB | - | 30,400 THB | 5,000 THB | 36,400 THB |
Import | 1,000 THB | - | 38,000 THB | 10,000 THB | 49,000 THB |
Certificate of Licensed Medical Device (Class 4) | |||||
Local Manufacture | 1,000 THB | - | 42,400 THB | 10,000 THB | 53,400 THB |
Import | 1,000 THB | - | 53,000 THB | 20,000 THB | 74,000 THB |
*** Once you have received the certificate of listed/notified/license medical device, you can place your medical device on the market.***
Step 6 : Post-marketing Obligation
You can view the post-marketing obligations for manufacturers and importers here
Step 7 : Advertisement Approval
Anyone who wishes to advertise a medical device must obtain advertising approval.